+380 63 419 0122
caslinkllc@gmail.com
10 Box (MOQ)
| Business Type | Exporter, Supplier, Retailer, Wholesaler, Distributor |
| Grade Standard | Pharm Grade |
| Form | Tablets |
| Usage | Clinical, Hospital, Personal, For Pain Killer |
| Click to view more | |
Preferred Buyer From
| Location | All Countries |
Product Details
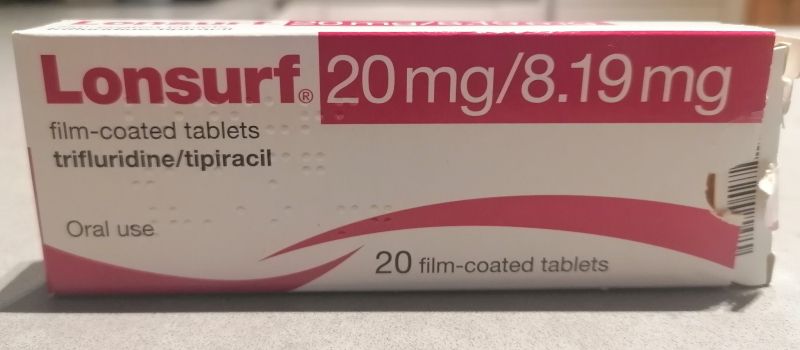
Lonsurf Tablets Name of the medicinal productPharmaceutical formFilm-coated tablet (tablet).Lonsurf 15 mg/6.14 mg film-coated tabletsThe tablet is a white, biconvex, round, film-coated tablet, with a diameter of 7.1 mm and a thickness of 2.7 mm, imprinted with ’15’ on one side, and ‘102’ and ’15 mg’ on the other side, in grey ink.Lonsurf 20 mg/8.19 mg film-coated tabletsThe tablet is a pale red, biconvex, round, film-coated tablet, with a diameter of 7.6 mm and a thickness of 3.2 mm, imprinted with ’20’ on one side, and ‘102’ and ’20 mg’ on the other side, in grey ink.Qualitative and quantitative compositionLonsurf 15 mg/6.14 mg film-coated tabletsEach film-coated tablet contains 15 mg trifluridine and 6.14 mg tipiracil (as hydrochloride).Excipient with known effectEach film-coated tablet contains 90.735 mg of lactose monohydrate.Lonsurf 20 mg/8.19 mg film-coated tabletsEach film-coated tablet contains 20 mg trifluridine and 8.19 mg tipiracil (as hydrochloride).Excipient with known effectEach film-coated tablet contains 120.980 mg of lactose monohydrate.For the full list of excipients, see section 6.1.**Therapeutic indicationsColorectal cancerLonsurf is indicated as monotherapy for the treatment of adult patients with metastatic colorectal cancer (CRC) who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents.Gastric cancerLonsurf is indicated as mono therapy for the treatment of adult patients with metastatic gastric cancer including adenocarcinoma of the gastrointestinal junction, who have been previously treated with at least two prior systemic treatment regimens for advanced disease.Posology and method of administrationLonsurf should be prescribed by physicians experienced in the administration of anticancer therapy.PosologyThe recommended starting dose of Lonsurf in adults is 35 mg/m2/dose administered orally twice daily on Days 1 to 5 and Days 8 to 12 of each 28-day cycle as long as benefit is observed or until unacceptable toxicity occurs (see section 4.4).The dosage is calculated according to body surface area (BSA) (see Table 1). The dosage must not exceed 80 mg/dose.If doses were missed or held, the patient must not make up for missed doses.Special warnings and precautions for useBone marrow suppressionLonsurf caused an increase in the incidence of myelosuppression including anaemia, neutropenia, leukopenia, and thrombocytopenia.Complete blood cell counts must be obtained prior to initiation of therapy and as needed to monitor toxicity, but at a minimum, prior to each treatment cycle.Treatment must not be started if the absolute neutrophil count is < 1.5 ×109/L, if the platelet counts are < 75× 109/L, or if the patient has an unresolved Grade 3 or 4 non-haematological clinically relevant toxicity from prior therapies.Serious infections have been reported following treatment with Lonsurf (see section 4.8). Given that the majority were reported in the context of bone marrow suppression, the patient’s condition should be monitored closely, and appropriate measures, such as antimicrobial agents and granulocyte-colony stimulating factor (G-CSF), should be administered as clinically indicated. In RECOURSE and TAGS studies, 9.4% and 17.3% of patients in the Lonsurf group respectively received G-CSF mainly for therapeutic use.Gastrointestinal toxicityLonsurf caused an increase in the incidence of gastrointestinal toxicities including nausea, vomiting and diarrhoea.Patients with nausea, vomiting, diarrhoea and other gastrointestinal toxicities should be carefully monitored, and anti-emetic, anti-diarrhoeal and other measures, such as fluid/electrolyte replacement therapy, should be administered as clinically indicated. Dose modifications (delay and/or reduction) should be applied as necessary (see section 4.2).Renal impairmentLonsurf is not recommended for use in patients
Looking for "Lonsurf 20mg Tablets" ?
Explore More Products